Last Updated:18 3月. 2014
Congenital heart disease
Background+ SHOW
The incidence of congenital heart disease (CHD) is 0.8 to 1 per 100 live births1)2). Of all cases of CHD, over a third are severe cardiac abnormalities, and these are the leading cause of infant death. Prenatal diagnosis of CHD improves neonatal morbidity and mortality; however, CHD is one of the most difficult congenital abnormalities to diagnose prenatally, and as such, a prenatal detection rate is still low.
Cause+ SHOW
5% of CHDs are related to single gene defects, 8% are related to chromosomal abnormality such as Down’s syndrome, and less than 1% are related to environmental factors such as maternal infection or exposure to drugs. Most of CHDs have no known causes .
Diagnosis+ SHOW
A fetal echocardiogram should be performed optimally between 18 and 30 weeks’ gestation by an obstetrician or pediatric cardiologist. Careful attention should be paid to the cardiac size and position, and the relative locations and size of the atrium, ventricle and major blood vessels. Some conditions are difficult to detect in utero, so not all heart problems can be ruled out.
General treatments+ SHOW
Once a diagnosis is made using a fetal echocardiogram, an explanation is provided regarding anticipated progress, treatment and prognosis. The patient should be allocated to a tertiary care center that can provide perinatal management. The delivery method will depend on the policies of the facility, with cesarean sections being planned for cases in which it is considered that intensive treatment will be required immediately after birth, or cases accompanied by arrhythmia. After delivery, a further echocardiogram or other examination is performed. CHDs dependent on the ductus arteriosus should begin intravenous treatment to maintain patency of the ductus arteriosus, and surgery is planned as required for the neonatal period. For conditions requiring surgery, the procedures are planned for implementation either all at once or over several operations, while observing the infant’s respiratory state and weight gain. Not all people with CHDs require treatment. Some may only need to be observed and visit their cardiologist.
Fetal cardiac intervention+ SHOW
Some fetuses with severe CHDs are at high risk for prenatal or neonatal death needed operation immediately after birth, others are needed staged univentricular palliation. The prognosis for such heart diseases is being improved by better prenatal diagnosis, perinatal management, neonatal management and progress in surgical treatment, but the long-term prognosis is still not at a satisfactory level. For this reason, fetal cardiac interventions for critical aortic stenosis have been performed since 2000, led by a Boston Children’s Hospital. Conditions in regard to which fetal therapy is currently implemented overseas include severe aortic valve stenosis, severe pulmonary stenosis, pulmonary atresia with intact atrial septum (PA/IVS), and hypoplastic left heart syndrome(HLHS) with intact or highly restrictive atrial septum.
It has been shown that releasing stenosis during the fetal stage for conditions resulting in major blood vessel valve stenosis during mid-pregnancy may facilitate the establishment of biventricular circulation, and trials have been conducted for this sort of fetal therapy. At present, balloon valvuloplasty is implemented overseas in regard to severe aortic valve stenosis, severe pulmonary stenosis and PA/IVS on patients between around 20 and 30 weeks’ gestation, and reports note that this has allowed the establishment of biventricular circulation after birth. Once the mother and fetus have been anesthetized, the fetus is adjusted into a position suited to centesis via the mother’s abdominal wall. Centesis is implemented from the ventricle apex in the direction of the stenosis under ultrasound observation, after which the balloon is inflated3)4).
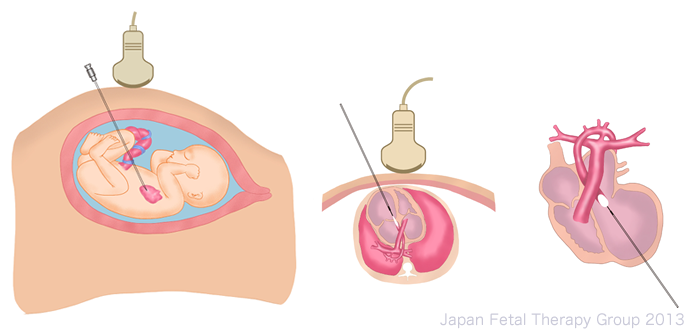
Since 2001, surgery to create interatrial communication has been implemented in utero in cases of HLHS with intact or highly restrictive atrial septum. Once anesthetized, a catheter is inserted from the right atrium to create a hole in the atrial septum, which is extended using the balloon. This hole sometimes closes again, and techniques such as the insertion of a stent have been used to counter this5).
Fetal therapy and prognosis+ SHOW
The use of balloon valvuloplasty in cases of severe aortic valve stenosis has been technically successful in between approximately 67 and 80% of cases, but biventricular circulation has been achieved in only between roughly 33 and 67% of these, with reports suggesting that around 10% of fetuses die in utero6)7)8). Balloon valvuloplasty is considered technically difficult with regard to cases of PA/IVS and severe pulmonary valve stenosis. There are few reports of cases in which it has been applied, but of these, 64% reported technical success9).
There have also still been few reports of surgery to create interatrial communication in cases of HLHS with intact atrial septum; however, 90% of reported cases have achieved technical success. There is a tendency for such cases to result in reclosure, however, and improved technique is therefore required. Reports indicate a 9.5% risk of in utero fetal death. The survival rate after birth is reported to be 58%6).
References+ SHOW
1) Hoffman Jl. Incidence of congenital heart disease: Ⅱ. Prenatal incidence. Pediatr Cardiol. 1995; 16: 155-165
2) Hoffman Jl, et al. The incidence of congenital heart disease. J Am Coll Cardiol. 2002; 39: 1890-1900
3) Tworetzky W, et al. Balloon dilation of severe aortic stenosis in the fetus: Potential for prevention of HLHS: Candidate selection, technique, and results of successful intervention. Circulation 2004; 110: 2125-2131
4) Marshall AC, et al. Results of in utero atrial septoplasty in fetuses with hypoplastic left heart syndrome. Prenat Diagn 2008; 28: 1023-1028
5) Arzt W, et al. Intrauterine aortic valvuloplasty in fetuses with critical aortic stenosis: experience and results of 24 procedures. Ultrasound Obstet Gynecol 2011; 37: 689-695
6) Rychik J, et al. The hypoplastic left heart syndrome with intact atrial septum: atrial morphology, pulmonary vascular histopathology and outcome. JACC 1999; 34: 554-560
7) McElhinney DB, et al. Current status of fetal cardiac intervention. Circulation 2010; 121: 1256-1263
8) McElhinney, et al. Predictors of technical success and postnatal biventricular outcome after in utero aortic valvuloplasty for aortic stenosis with evolving hypoplastic left heart syndrome. Circulation 2009; 120: 1482-1490
9) Tworetzky, et al. In utero valvuloplasty for pulmonary valve atresia with hypoplastic right ventricle: techniques and outcomes. Pediatrics 2009; 124: 2008-2014

